Alfa Wassermann continuous flow ultracentrifuges meet all the demands of cGMP manufacturing for viral vaccines and viral vector gene therapy products. Over forty years of Alfa Wassermann’s experience has led to the development of a sophisticated and robust ultracentrifuge suitable for running upward of 3000 operational hours a year.
In 1967 this technique was made available commercially by Electro Nucleonics Inc (now Alfa Wassermann). Since then the world leading pharmaceutical manufacturers are utilizing the KII ultracentrifuge to produce purifed Influenza vaccine on a large scale as well as Meningitis, Rabies, Hepatitis B and other vaccines.
Drive Technology
Alfa Wassermann provides both the traditional Air Drive or an electric motor to provide the driving force to spin the rotor in the rotor chamber.
The Air Drive is traditionally used in high usage processes where robustness and durability are required.
The ‘e-drive’ electric motor has been developed for stand-alone use with only an electrical supply required. The noise emissions of the e-drive KII are very low and therefore suitable for laboratory use.
System Control
A PLC or PC-based control runs the proprietary AWST control interface console software. Critical lubrication and speed control systems are built in with alarms con gured to protect the product and machine from harm.
Control Interface
A clear windows based HMI allows for easy operator interaction. Input of data is via keyboard and touch screen. On screen visual and audible alarms enable unattended operation.
Monitoring of:
- Speed
- Vacuum
- Lubrication Flow
- Coolant Flow
- Lubrication Level
- Coolant Level
- Refrigerator
- Temperature Rotor
- Temperature Monitoring
- Operations:
- User ID
- Password
- Batch ID
- Rotor ID
- Batch Time
- System Run Time
- Rotor Run Time
Data Monitoring
Continuous data monitoring to a named batch le throughout the run can
be reviewed on screen and is saved to the system before being transferred to a remote location. User con gurable logging allows only process critical parameters to be trended. Batch data can be transferred to a process control unit or sent to a remote printer.
Validation
The system is fully validated for use in cGMP manufacturing processes and is the choice for vaccine manufacturers. The control console regulates and controls all necessary process uids, safety features allow for unattended operation, password security enables compliance to 21 CFR Part 11 and GAMP.
Scale Up & Scale Down of Processes
Density gradient ultracentrifugation allows concentration and purification in a single step, reducing the total number of process steps and process time and therefore increasing the overall yield and production capacity. Selection from a range of linear scalable rotor cores enables scale up and scale down of process parameters. The K3 linear scale cores retain the same separation path but differ in rotor volume, which allows for the exact same process puri cation to be achieved at production scale as well as at pilot scale volumes.
To start purification using the ultracentrifuge clarified harvest material can be processed directly, with no concentration or buffer exchange required. Bulk harvest can be processed without clarification using the integrated pre clari er K6 rotor assembly.
The simple fluid path of the ultracentrifuge rotor creates a low shear environment which helps retain the viability of virus particles during downstream processing. Use of sucrose as a density gradient matrix is widely applied and cost effective for virus like particle puri cation and can easily be removed in subsequent processing.
KII Rotor Specification
The rotor assembly is composed of a tubular bowl and two end caps which are made of Titanium Alloy. These create the housing of the rotor assembly and contain within the rotor core. This assembly forms the six flow channels down which process material flows during operation.
- Maximum Speed and Centrifugal Force: 40 500 rpm, 121 200 xg
- Flow Rate Range: 0 l/h (batch) to 60 l/h
- Process Volume: 5 l to 200 l continuous flow
| Rotor Type |
Application |
Parameters |
Capacity with core, L |
Dimensions |
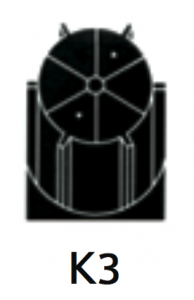 |
For separation using isopycnic banding techniques with viral particles, virus like particles or nano-spheres. The basis of separation is the difference in buoyant densities of the particles being separated. |
At 40 500 rpm:Rmax: 121 200xg
Rmin: 100 000xg
|
3.2 |
Diameter:Max:130 mm / 5.2”
Min: 110 mm / 4.3”
Path Length:11 mm / 0.45”
|
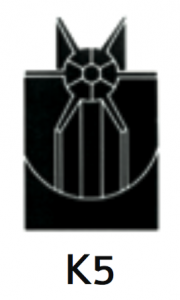 |
For separation using rate zonal techniques. Separation is based on the sedimentation rates of material being separated. Sequential rate zonal and isopycnic banding centrifugation permits a two dimensional separation to be made on the basis of both particle size and density. |
At 40 500 rpm:Rmax: 121 200xg
Rmin: 38 500xg
|
8.4 |
Diameter:Max: 130 mm / 5.2”
Min: 42 mm / 1.6”
Path Length:45 mm / 1.7”
|
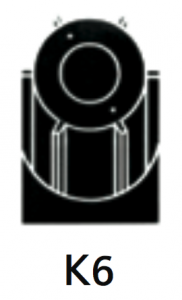 |
For separation using isopycnic banding techniques with viral particles, virus like particles, nano-spheres. Integral pre-clarifier allows the initial capture of heavy particles such as whole cells or cell debris, and eliminates the need for a pre-clarification step. |
At 40 500 rpm:Rmax: 121 200xg
Rmin: 100 000xg
Pre-clarifier:
Rmax: 53 900xg
Rmin: 49 000xg
|
3.2Pre-clarifier:0.17
|
Diameter:Max: 130 mm / 5.2”
Min: 110 mm / 4.3”
Path Length:11 mm / 0.45”
Pre-clarifier: Diameter:
Max: 58 mm / 2.3”
Min: 23 mm / 0.94”
|
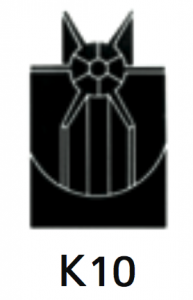 |
Separation of large volumes of solids by isopycnic banding techniques.The basis of separation is the difference in buoyant densities of the particles being separated. |
At 40 500 rpm:Rmax: 121 200xg
Rmin: 38 500xg
|
8.0 |
Diameter:Max: 130 mm / 5.2”
Min: 53 mm / 2.1”
Path Length: 39mm/1.5”
|
 |
Separation of low molecular weight sub-cellular components.Very short sedimentation path permits the separation of extremely small particles. |
At 40 500 rpm:Rmax: 121 200xg
Rmin: 121 100xg
|
0.38 |
Diameter:Max: 130 mm / 5.2”
Min: 129.5 mm / 5.2”
Path length:0.5 mm / 0.02” |
Applications
The ultracentrifugation process typically uses density gradient continuous flow ultracentrifuge for the purification and concentration of virus particles. The table below describes the general conditions for separation and purifcation of a range of viral products.
|
Influenza Vaccine |
Rabies Vaccine |
Hepatitis B Vaccine |
Adenovirus Vector |
| System |
KII |
KII |
KII |
pKII |
| Rotor |
K3 3,2 l |
K3 3,2 l |
K5 8,4 l |
PK3 1,6 l |
| Flow rate |
20 l/h |
16 l/h |
Batch Operation |
10 l/h |
| Gradient |
0-55% (w/w) sucrose |
0-65% (w/w) sucrose |
0-55% (w/w) sucrose |
0-40% (w/w) Nycodenz |
| Volume |
150 l |
40 l |
5 l |
20 l |
| Capture |
95% |
95% |
100% |
95% |
| Recovery |
70% |
90% |
80% |
70% |
| Purification factor |
x 50 |
x 90 |
x 10 |
x 20 |
Zonal Density Gradient Reorientation
Gradient materials are loaded while the rotor is at rest, the flow is stopped and a linear density gradient is formed while the rotor is accelerating to a set speed. Following a reorientation phase the gradient transitions from horizontal to vertical in the rotor. After this transition occurs then the rotor can be accelerated to set speed while in a continuous flow operation.
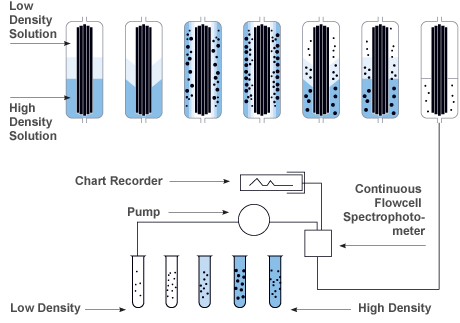
| Step |
Process |
| 1 |
The density gradient is loaded into the rotor while it is at rest. |
| 2 |
As the motor is gradually accelerated, the gradient reorients itself vertically along the outer wall of the rotor assembly. |
| 3 |
Once at operating speed, sample fluid is pumped into the rotor on a continuous flow basis. |
| 4 |
The sample particles sediment radially into the gradient of increasing density. They eventually band (iso-pycnically) in cylindrical zones where the gradient density equals a particle's buoyant density. |
| 5 |
At the end of the run, the rotor is decelerated to rest. |
| 6 |
The gradient reorients itself to the original position without disturbing the particle bands. |
| 7 |
The banded particles are now ready to be unloaded. Fractions are collected using air or water pressure and a pump to control flow. |
System technical data
| Parameter |
Specification |
| Rotor material |
Titanium Alloy |
| Core material |
Noryl®, PEEK or Titanium |
| Drive Technology |
Electric Motor or Air Drive |
| Process Flow Rates & Pressures |
Up to 60 l/h and 1 bar max. |
| Process Temperatures |
+4...+30°C +/- 2°C |
| Process Volumes |
200 liters per run |
| Maximum Speed |
40 500 rpm +/- 100 rpm |
| Maximum Centrifugal Force |
121 200 xg |
| Process Connections |
3A Sanitary Fittings |
| Material (process wetted) |
316LSS, Titanium, Noryl®, Te on, 440C SS |
| Gaskets |
USP Class VI |
| Lubricants |
WFI & Pharmaceutical Grade H1 Hydraulic Oil |
| Noise emissions |
Compliant with CE; suitable for use in laboratory environments |
| Control Panel Specifications |
IP65 HMI with touch screen for access to; virtual controls, LED alarms, on screen gauges and trending. The display units are con gurable. |
| Interface Language |
English, French, German, Italian, Spanish |
| Regulatory Compliances |
21 CFR part 11, GAMP, cGMP |
| Manufacturing Compliances |
CE, ISO 13485 Registered Company |
| Rotor Handling |
System integrated lift and rotor cart for storage |
| Warranty |
System 1 year including rotor |



















